In March 2022, SIMCor has finalised its methodology for generation and validation of virtual cohorts of animal models and human patients of aortic valve disease (AVD) and heart failure (HF). This has been reported in four relevant deliverables, all publicly available in open access: (1) D7.1 – Definition of model output, (2) D7.2 – First version of the simulation models, (3) D7.3 First version of the definition of the input space and (4) D7.4 – Sensitivity and uncertainty quantification toolbox.
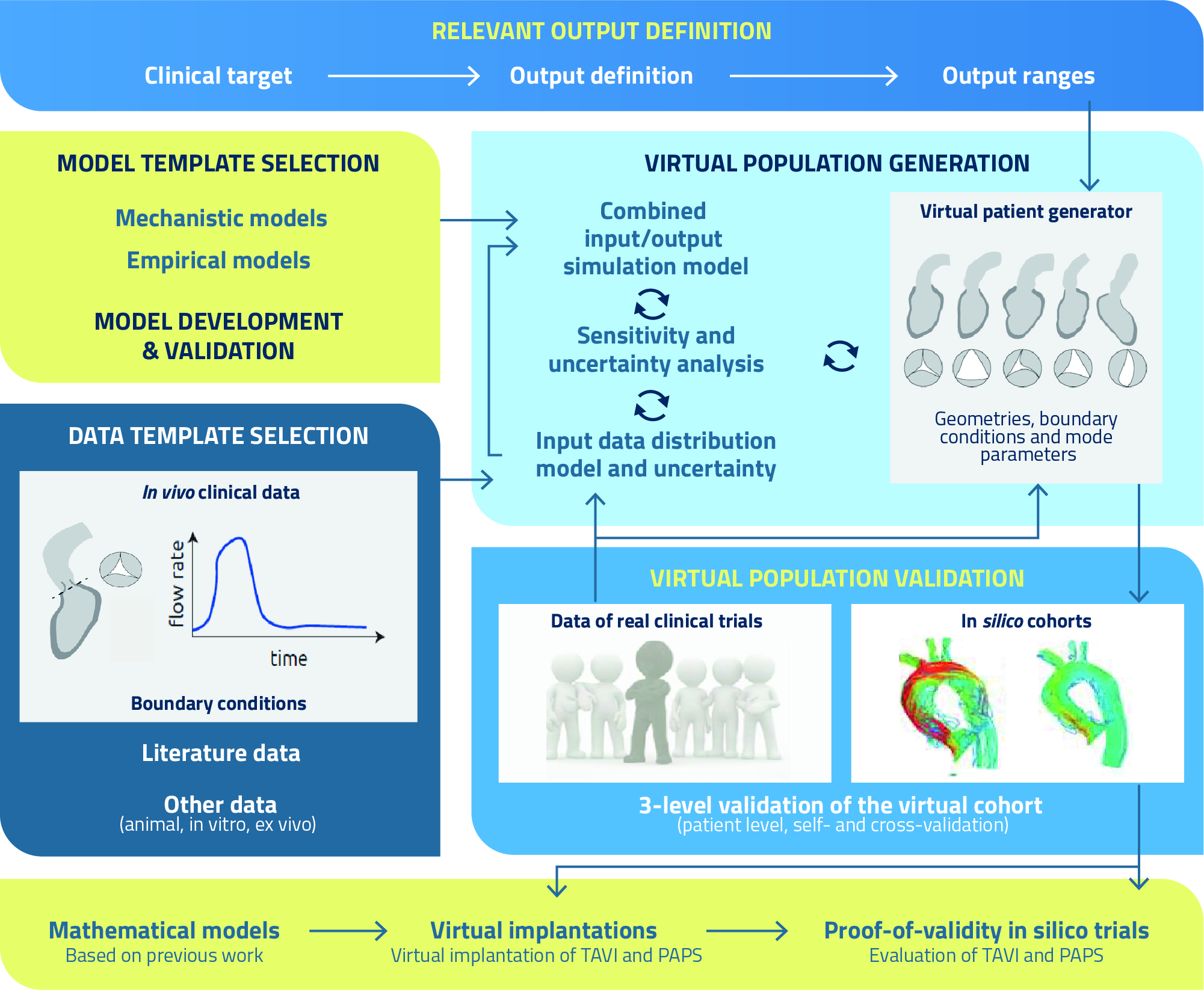
One of the primary aims of SIMCor is the development and validation of virtual patient cohorts based on features of the true population. These models can integrate patients’ input data into engineering concepts (e.g., pressure, flows, axial forces) by means of established physiological and physical laws, thus allowing to create virtual patients with meaningful physiological behaviour by varying their input parameters.
However, non-physiological candidates might occur due to non-physiological combinations of input parameters. Fortunately, this can be resolved by defining proper model output criteria and their ranges: each candidate that has output of interests outside the predefined ranges can be excluded from our virtual cohorts. These outputs thus serve as criteria to filter out non-physiological virtual patients from our cohort.
The entire process can be summarised as follows:
- Definition of the outputs of interest: definition of the output ranges that are representative for the geometry, haemodynamics and physiology of AVD and HF patients, as well as the clinical targets, i.e., engineering metrics related to the clinical performance of ranscatheter aortic valve implantation (TAVI) and pulmonary artery pressure sensor (PAPS) implantation, respectively, such as paravalvular leakage (TAVI), device migration and vessel perforation (PAPS), and thrombosis (both TAVI and PAPS), along with their correlations and joint probability functions.
- Definition and selection of model templates: develop or select models that can accurately predict the cardiovascular physiology of patients before and after TAVI and PAPS implantation, including the identification of an optimal balance between the level of complexity (i.e., number of model parameters) and uncertainty.
- Data template selection: definition of data distributions serving as input for the physics-based models, based on data collections, quantification of distributions and selection of the most relevant ones by sensitivity analysis, which will constitute the input space for the virtual cohort generator.
- Virtual cohort generation: sampling of the input space to be used as input for simulation models, resulting in an output and related uncertainty; if the output realisation is within the predefined output range, the sample is added to the virtual cohort, and this step is repeated until the full range of the defined output ranges is properly covered, i.e., the output statistics of the virtual cohort mimics the real output statistics.
- Virtual cohort validation: (a) patient-specific validation, where the model-derived metrics are validated on a patient-specific level to demonstrate that virtual simulations mimic real patient features; (b) self-validation based on data of two subsets of a real medical device clinical trial, to evaluate whether the model-generated cohort is able to accurately reproduce the observed intervention statistics; (c) cross validation, where the effect measure of a virtual cohort that consists of statistically similar patients is simulated and compared with the effect measure of the truly observed effect of the real clinical trial based on retrospective randomised clinical trial data.